A typical large tree can absorb up to 40 kilograms of carbon dioxide from the air in a year. Now scientists at UC Berkeley say they can do the same job with less than half a kilogram of fluffy yellow powder.
The dust is designed to trap the greenhouse gas in its microscopic pores, then release it when it’s ready to go somewhere where it can’t contribute to global warming. In tests, the material was still in good shape after 100 such cycles, according to a study published Wednesday in the journal Nature.
“It performs beautifully,” said Omar Yaghi, a lattice chemist at UC Berkeley and senior author of the study. “Based on the stability and behavior of the material now, we think it will go to thousands of cycles.”
Called COF-999, the dust can be placed in the types of large-scale direct air capture plants that are starting to come online to reduce the amount of carbon in the atmosphere.
Keeping the concentration of atmospheric carbon dioxide below 450 parts per million is necessary to limit global warming to 2 degrees Celsius above pre-industrial levels and prevent some of the most dire consequences of climate change, scientists say. Measurements taken at the Mauna Loa Observatory in Hawaii show that CO2 levels are currently around 423 ppm.
“You have to get CO2 out of the air — there’s no way around it,” said Yaghi, who is also chief scientist at the Bakar Institute for Digital Materials for the Planet in Berkeley. “Even if we stop emitting CO2, we have to remove it from the air. We have no other option.”
Klaus Lackner, founding director of the Center for Negative Carbon Emissions at Arizona State University, agreed that direct air capture will become an important tool for sequestering carbon and cooling the planet once significant hurdles are overcome. Advances in the new study may help, he said.
“They are opening a door to a new family of approaches,” said Lackner, who was not involved in the research.
When viewed under a scanning electron microscope, the dust resembles tiny basketballs with billions of holes, said study leader Zihui Zhou, a materials chemist working on his Ph.D. at UC Berkeley.
The structures are held together by some of the strongest chemical bonds in nature, including those that turn carbon atoms into diamonds. Compounds called amines are attached to the scaffolds.
When air flows through structures, most of its components pass through undisturbed. But amines, which are basic, capture carbon dioxide, which is acidic.
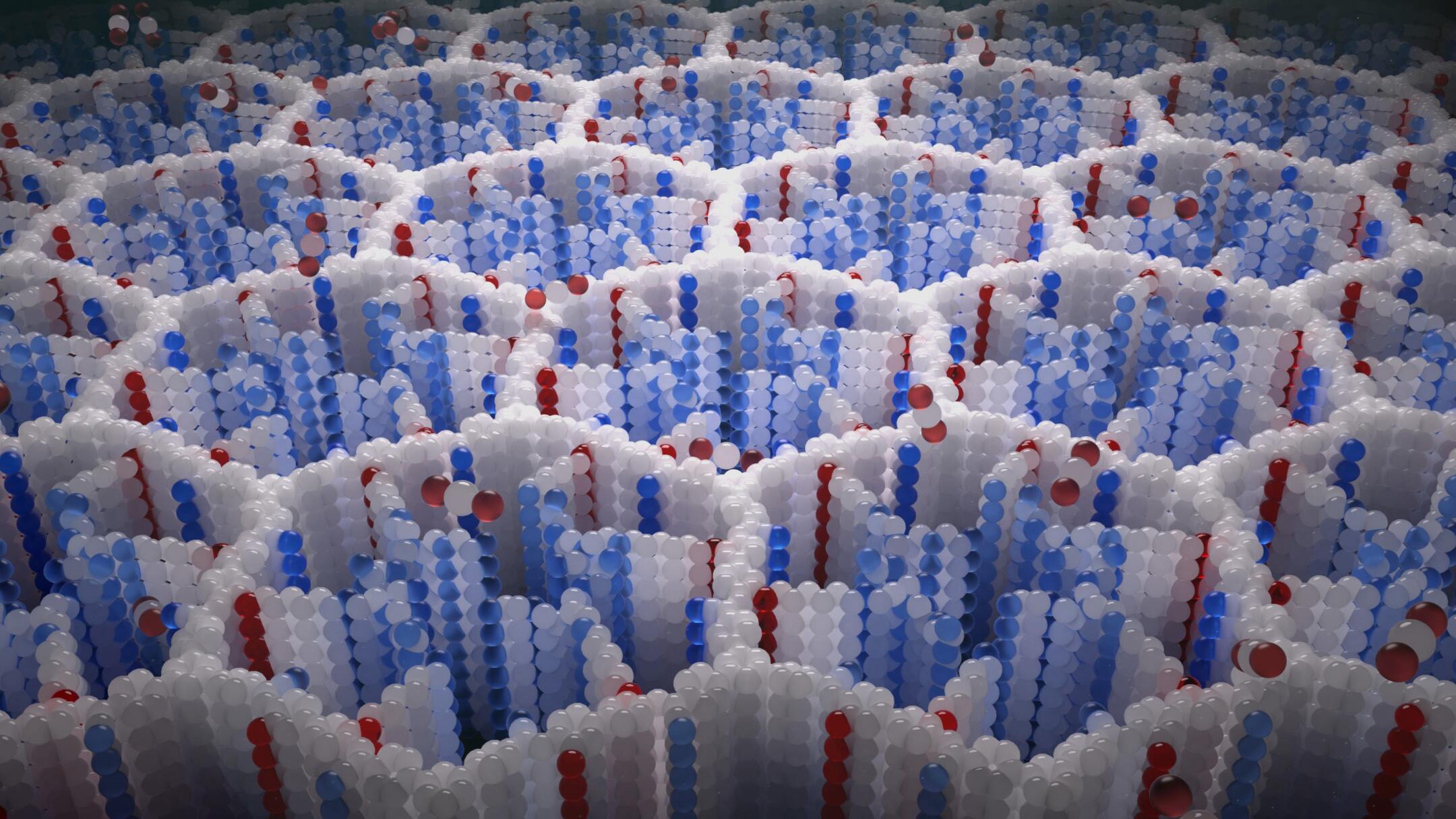
An illustration of the structure of COF-999, with pores that trap carbon dioxide molecules.
(Chaoyang Zhao)
Those CO2 molecules will stay put until scientists release them by applying heat. They can then clean them up for storage, most likely by pumping them deep underground, Zhou said.
Once the carbon dioxide is removed from the dust, the whole process can start again.
To test COF-999’s carbon scavenging capabilities, the researchers packed the powder into a stainless steel tube about the size of a straw and exposed it to Berkeley’s outdoor air for 20 consecutive days.
As it entered the tube, Berkeley’s air contained CO2 in concentrations ranging from 410 ppm to 517 ppm. When it came out the other side, scientists could detect no carbon dioxide at all, Zhou said.
The powder has several advantages over other materials, according to its creators.
Its porous design increases its surface area, which means more places to hold CO2 molecules. As a result, it captures carbon dioxide at a rate that is “at least 10 times faster” than other materials used for direct air capture, Zhou said.
Team members have continued to make improvements and they are on track to double its capacity in the coming year, Yaghi added.
Another plus is that COF-999 will release its hold on CO2 when it is heated to about 140 degrees F. Comparable materials must be heated to 250 degrees F to release the carbon, Zhou said.
The powder is also more durable. Zhou said the team tested a newer version that ran for 300 cycles before the experiment ended.
Lackner said that was a promising sign.
“Putting out 100 cycles and not seeing any deterioration suggests you can do thousands of cycles,” he said. “Whether you can get hundreds of thousands of cycles, we don’t know.”
Deploying it on an industrial scale will require designing some kind of large metal box through which air can pass without blowing away all the dust, Zhou said. Those boxes would have to be stacked together in quantities that evoke a modern chemical or oil plant.

Towering fan and tray structures capture carbon dioxide inside a direct air capture plant in Tracy, California, which opened last year.
(Paul Kuroda/For the Times)
Yaghi said a version of COF-999 could be ready for direct air capture plants within two years. He could not estimate how much bulk production would cost, but said he does not require any expensive or exotic materials.
Yaghi founded a company, Irvine-based Atoco, to commercialize his research on carbon capture and other technologies. Atoco helped fund the new study. (Other financial backers include Bakar Institute and King Abdulaziz City for Science and Technology.)
In addition, UC Berkeley has filed a patent application for COF-999, which names inventors Yaghi and Zhou.
Lackner said the whole process of direct air capture would have to become “10 times cheaper than it is now” before it could make a real dent in the hundreds of billions of tons of carbon dioxide that scientists would like to capture. swept away by the atmosphere.
A material that is more efficient at capturing CO2 would help, but Lackner said he spends more time worrying about issues like the heat lost when temperatures rise to harvest the carbon so it can be injected underground.
“There are a thousand things that feed into this,” he said.
Bulletin
Toward a more sustainable California
Pick up Boiling Point, our newsletter exploring climate change, energy and the environment, and be part of the conversation – and the solution.
You may occasionally receive promotional content from the Los Angeles Times.